A CASE OF POSTOPERATIVE COMPLICATION OF SURGICAL MITRAL VALVE REPLACEMENT.
Amin H. Karim MD
CASE:
A 71 year olf Caucasian male with history of hypertension and hyperlipidemia, and chronic atrial fibrillation, developed non-rheumatic mitral valve regurgitation secondary to mitral valve prolapse, which had gradually progressed over a decade years to severe regurgitation, accompanied by moderately severe tricuspid regurgitation. Patient tolerated the valvular lesions for many years till he became symptomatic with NYHA Class III dyspnea and agreed for intervention.
CARDIAC MRI: In November 2024, cardiac MRI revealed bi-leaflet mitral valve prolapse, with severe left atrial enlargement and moderate tricuspid regurgitation. The global ejection fraction was 65% with biventricular dilatation, and basal and mid inferolateral wall scarring. Mitral and tricuspid annulus were dilated.
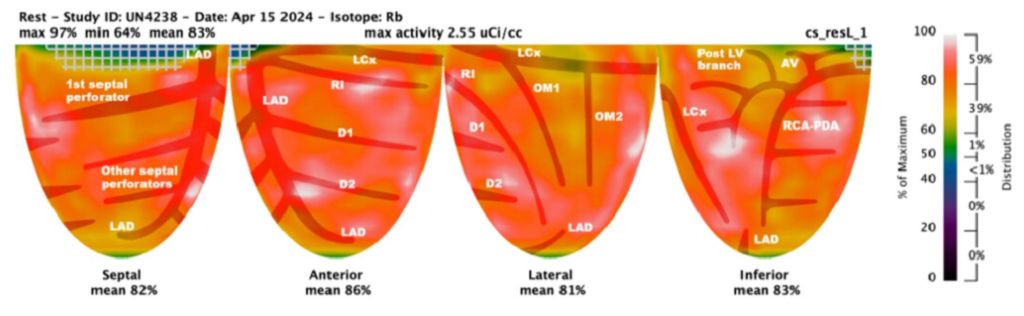
TWO DIMENSIONAL ECHOCARDIOGRAM: On Echocardiogram, right ventricular function was low normal. Left ventricular ejection fraction was normal. There was mitral valve prolapse due to myxomatous degeneration, severe mitral regurgitation with regurgitation fraction of 51% and estimated regurgitant volume of 56 ml. Pulmonary artery pressures were normal. Cardiac catheterization showed normal coronaries
With a low BMI and good overall health, he was felt by the cardiovascular surgeon to be low risk for surgery and MitraClip therefore not warranted.
MITRAL VALVE REPAIR SURGERY:
Mitral valve repair with a 34 mm physio 2 flexible annuloplasty ring; Intra-aortic balloon pump; Tricuspid valve repair with a 28 mm triad rigid ring; Biatrial maze utilizing the encompass clamp, RF clamp and cryoprobe; Left atrial appendage ligation with a 45 mm atrial cure mini atrial clip:
Following mitral valve repair there was no mitral regurgitation with a long segment of coaptation beneath the annular plane. The transmitral gradient was 1 mm Hg. Similarly following tricuspid valve repair there was no regurgitation with the trans tricuspid valve gradient of 1 mm.
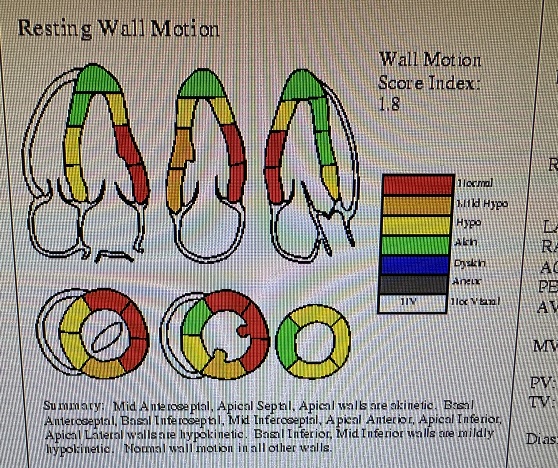
Following bi-atrial maze procedure, the patient converted to sinus rhythm. However, when the cardio pulmonary bypass was reversed and the patient was being closed, he went to ventricular fibrillation followed by defibrillation. Frequent PVCs were observed. Intravenous amiodarone and magnesium were started; His blood pressure started to drop and vasopressors were increased and intra-aortic balloon placed. His hemodynamics improved and was watched in the OR with echo. There was inferior wall hypokinesis. He was maintained on low dose dobutamine, vasopressin and norepinephrine. His global ejection fraction was reasonable; chest was closed, but before he could be transferred out of OR he developed ventricular fibrillation again. His chest was opened and direct cardiac massage and cardioversion done with return of circulation. ECMO (Extracorporeal Membrane Oxygenation) was initiated. He was transferred to the cardiac cath lab and underwent emergency coronary angiography.
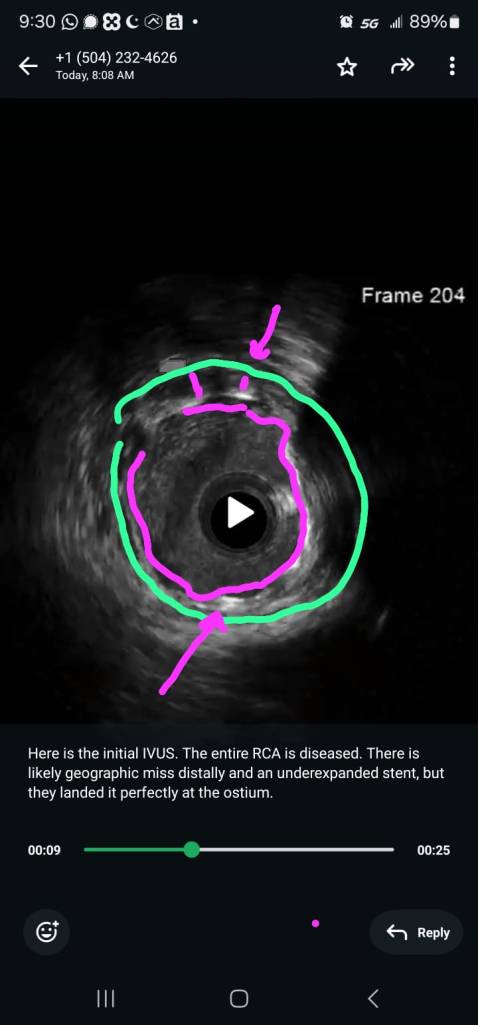
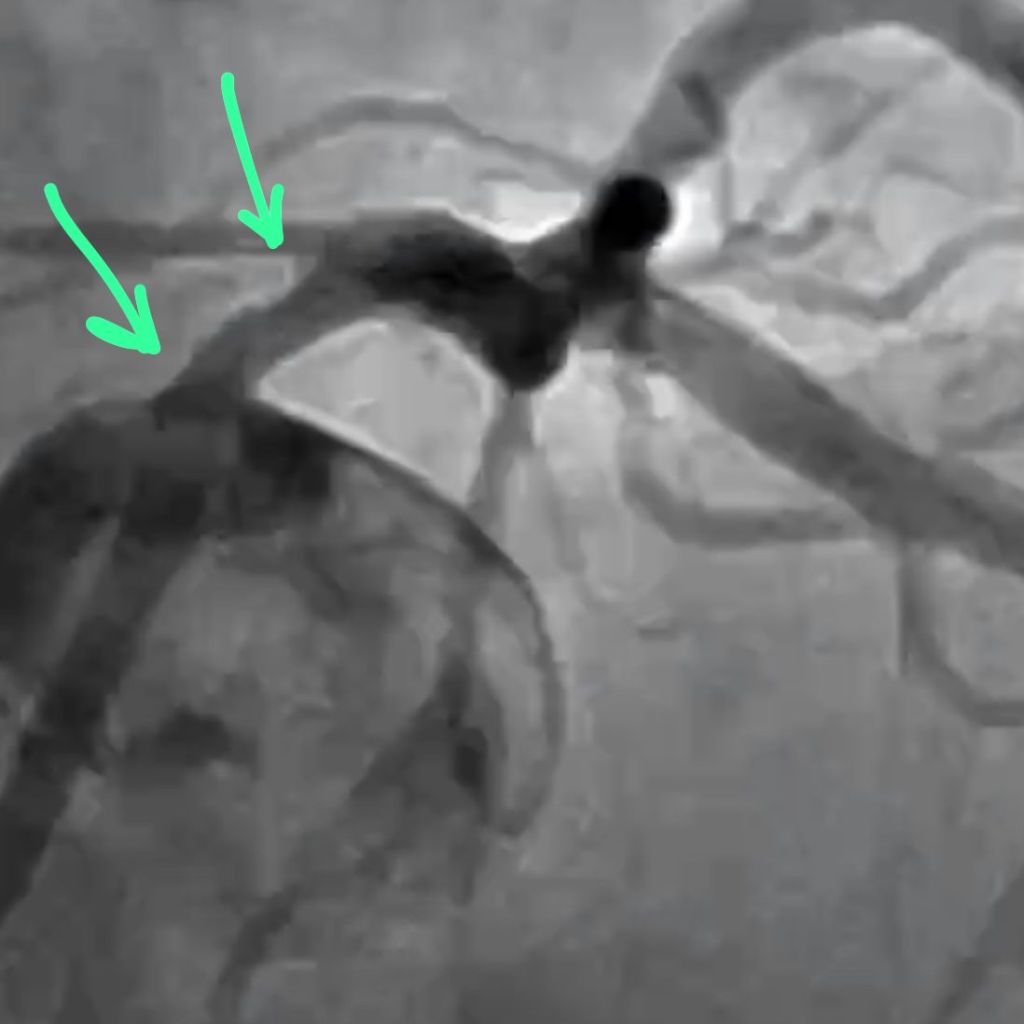
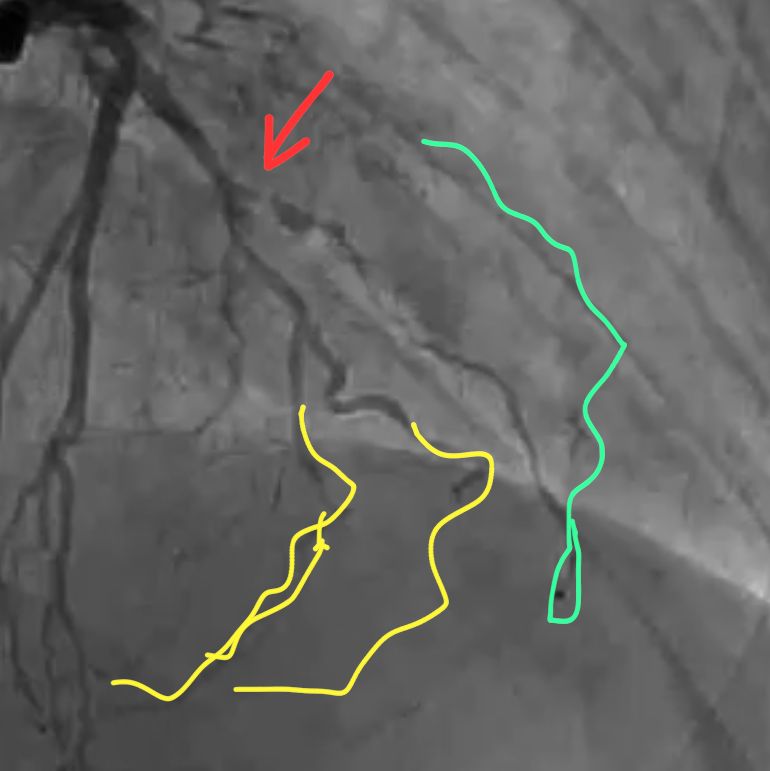
CORONARY INTERVENTION: Coronary angiography showed the dominant left circumflex was occluded in the mid potion.
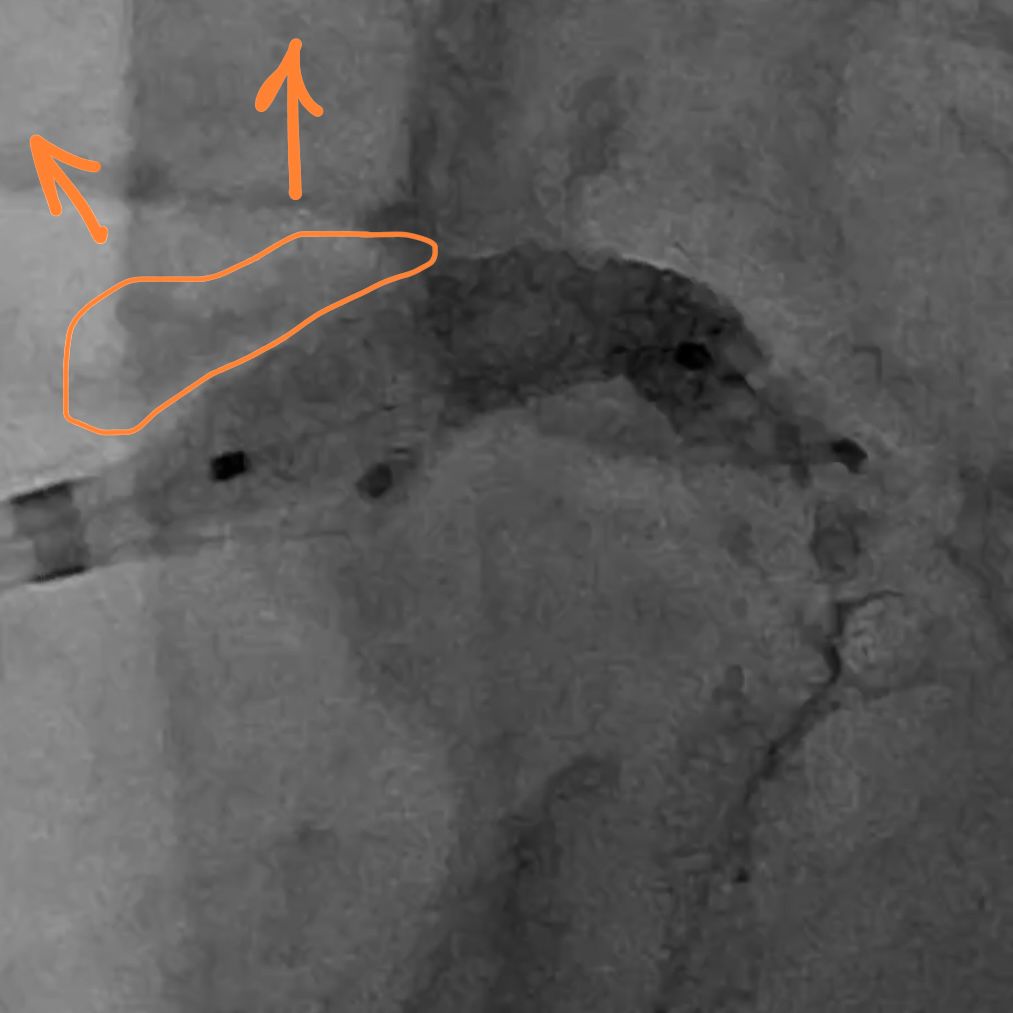
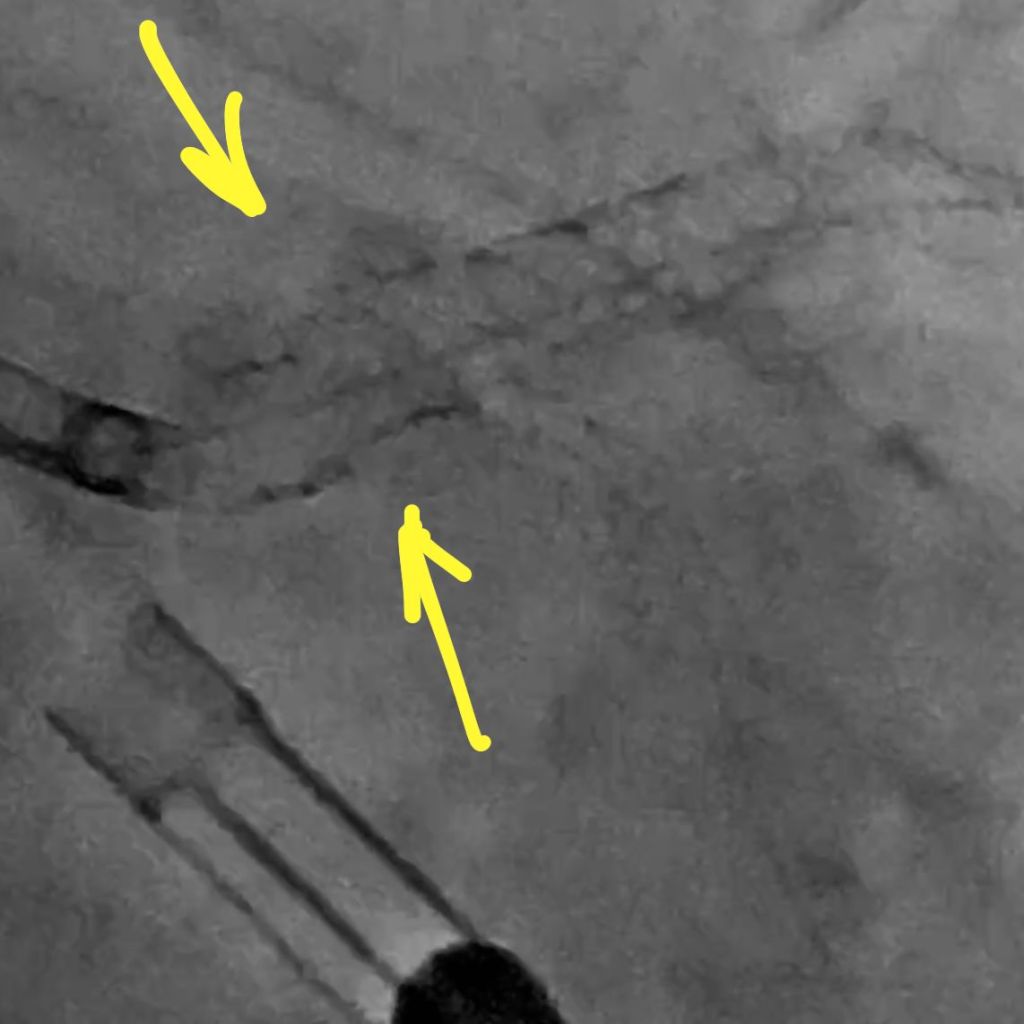
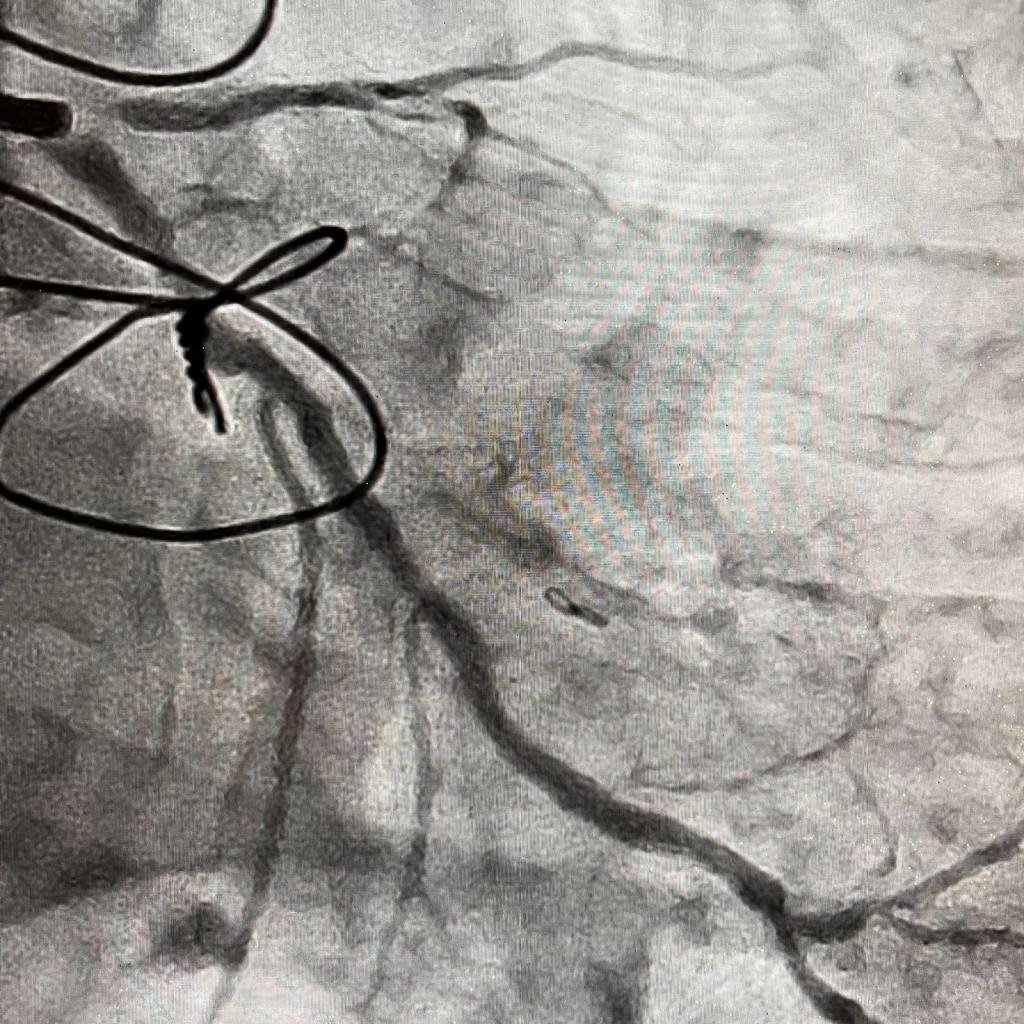
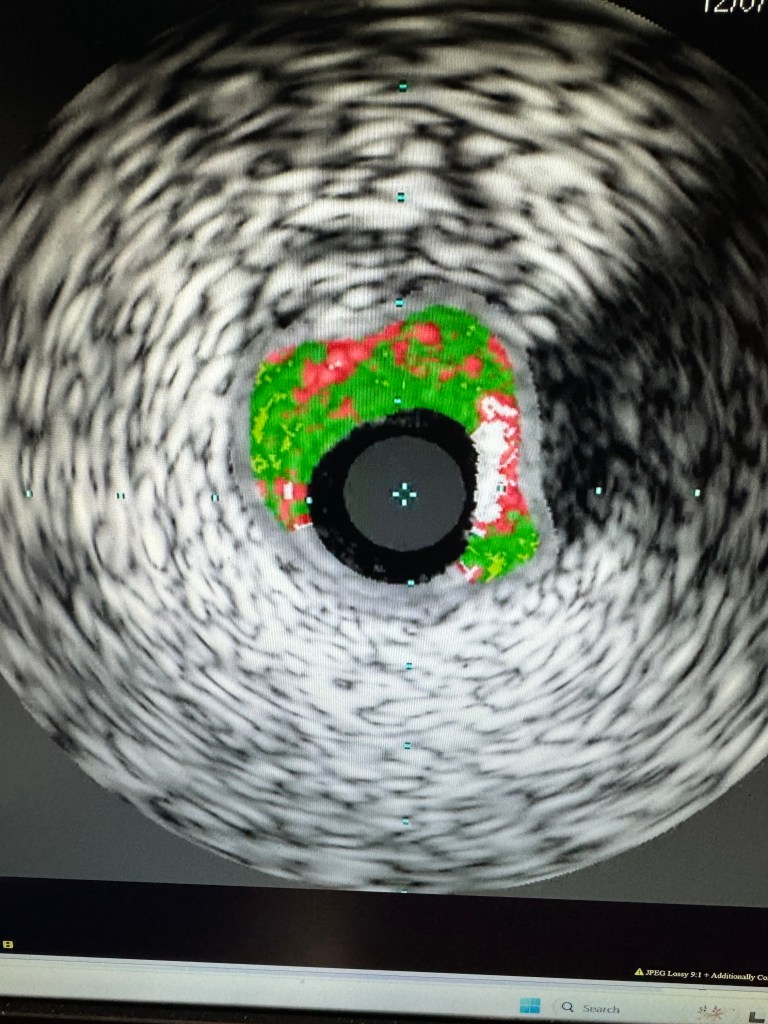
IVUS showed possible edema around the left circumflex and it was felt that the circumflex was occluded due to pressure from the mitral valve ring. Stent was placed with recanalization. Impella was placed in the cath lab and the intra-aortic balloon was removed.
Intavascular Ultrasound (IVUS) shows the edema/hematoma? aeound the left vircumflex artery.
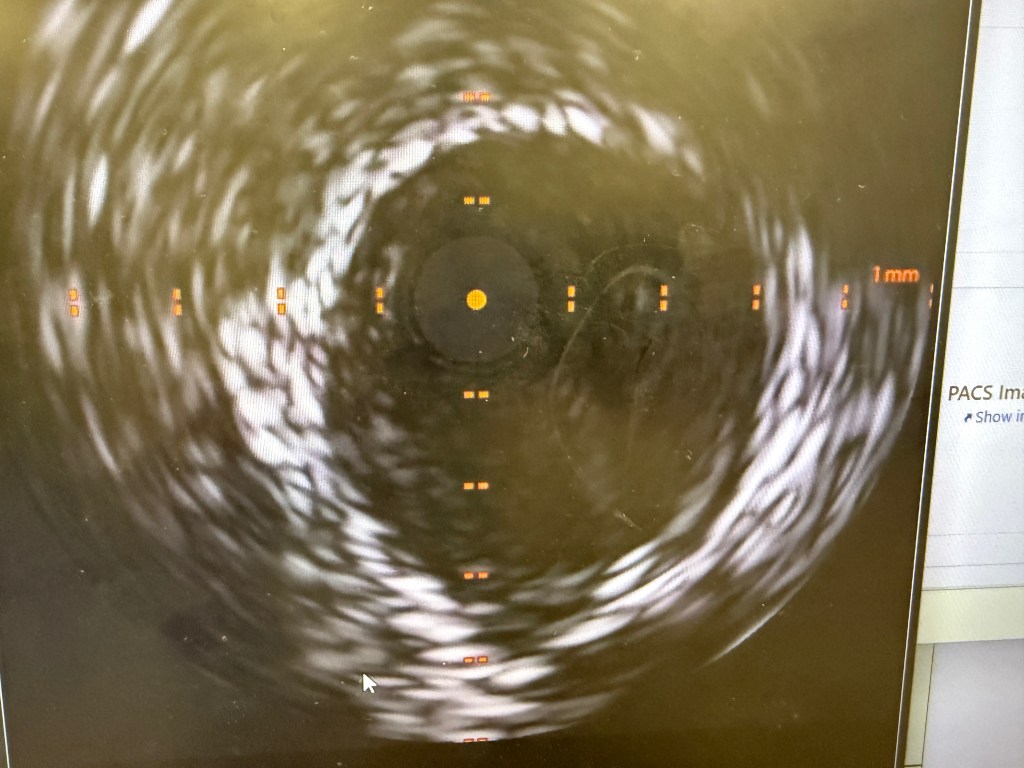
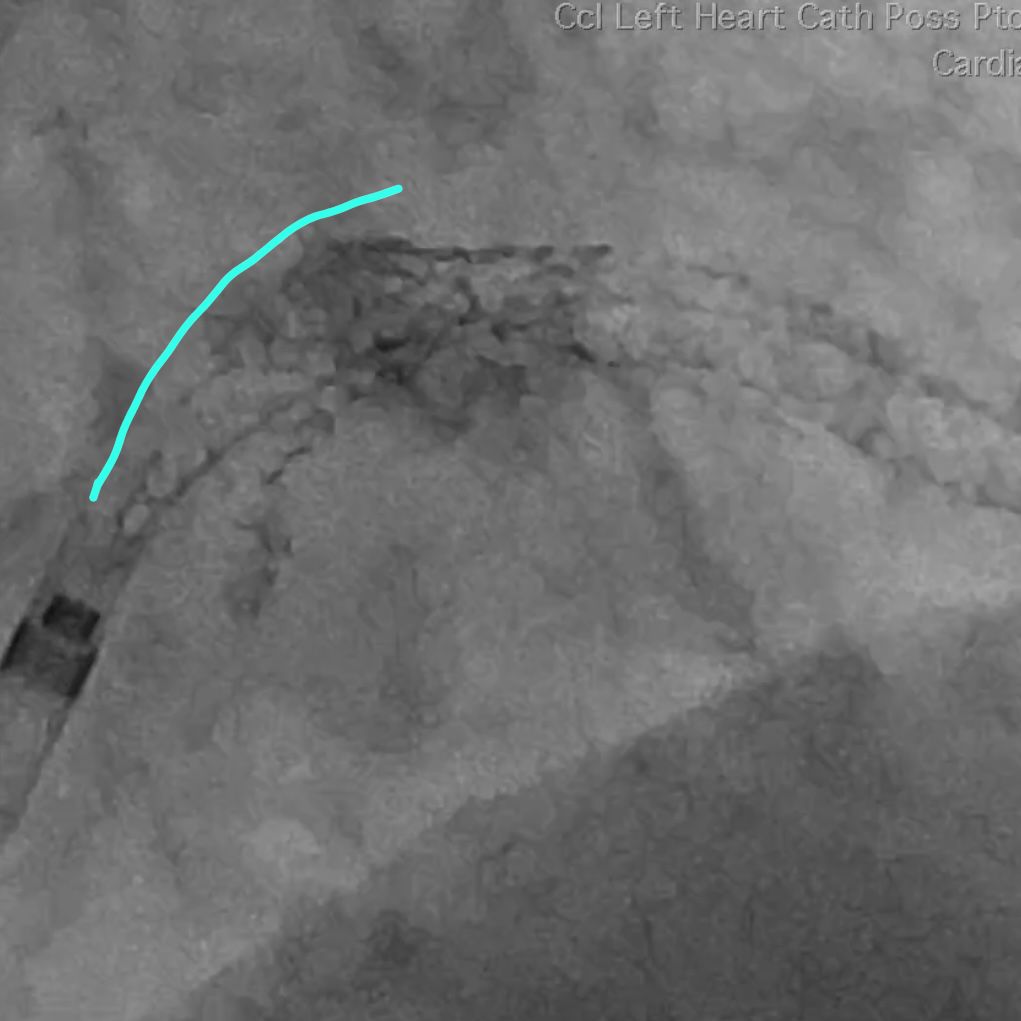
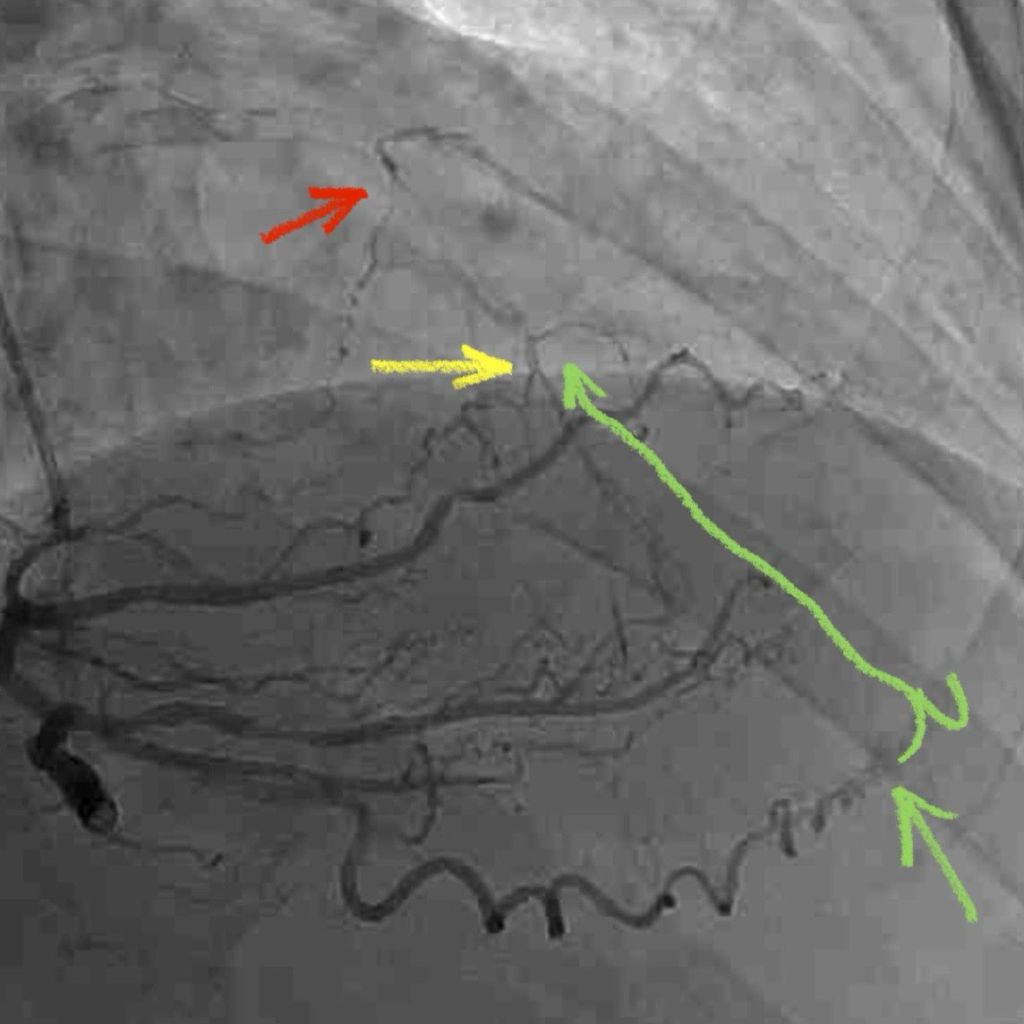
A 3.5 mm x 32 mm Synergy drug eluting stent was placed in the mid circumflex coronary artery with a good result. The intra-aortic balloon was removed.
INTENSIVE CARE UNIT: Patient remained with supported blood pressure, with severe anemia needing multiple blood transfusions. Transesophageal echo showed severely depressed right and left ventricular systolic function.
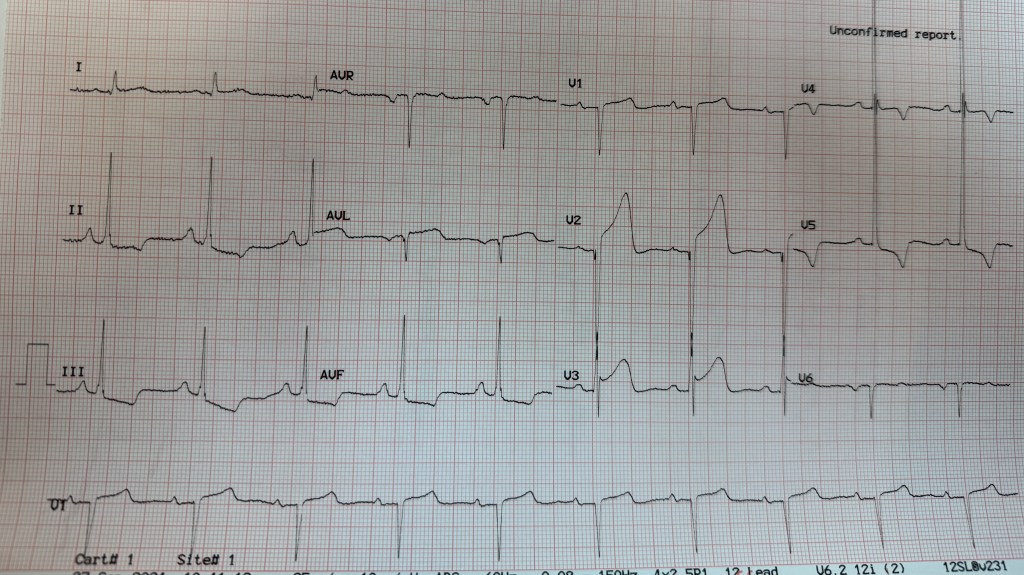
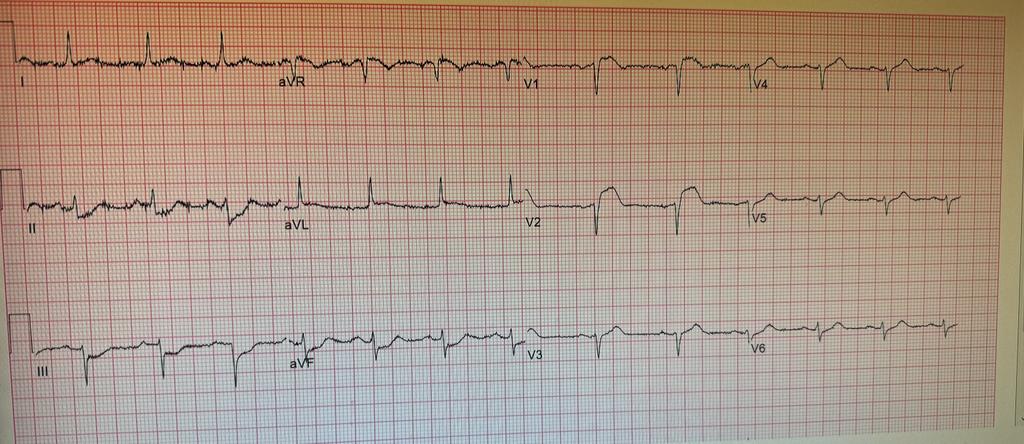
Patient was taken back to O.R. for exploration and washout. On return to ICU noted to have ST elevation in lead VII. Taken back to cath lab and coronary angiography done showing that the circumflex stent was patent.
FOLLOWUP: 10 days after mitral and tricuspid valve repair and coronary intervention, patient is off ECMO, and on Impella support. He is awake but does not follow commands. His global ejection fraction on echocardiogram is mildly depressed (40-45%) with trace of mitral and tricuspid regurgitation.