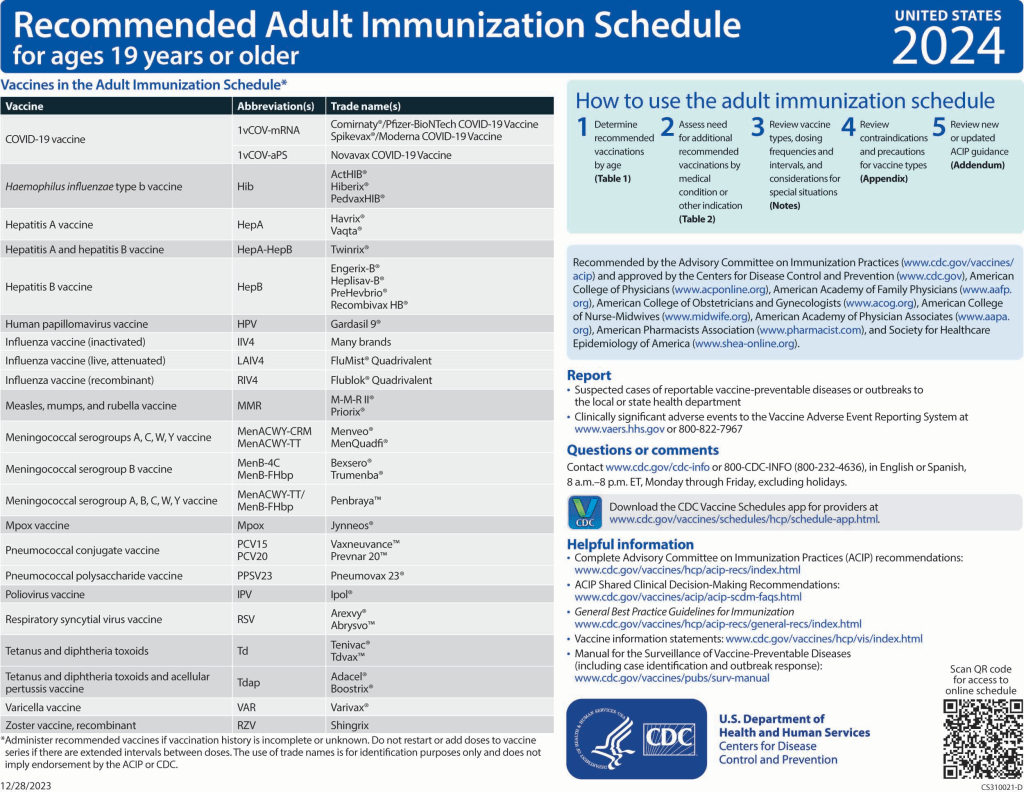
Changes to the 2024 Adult Immunization Schedule
COVID-19 vaccination (3). The COVID-19 vaccination section was updated to reflect the new COVID-19 vaccination recommendations that were approved during an ACIP public meeting held on 12 September 2023. All adults are now recommended to receive at least 1 dose of the updated (2023–2024 Formula) COVID-19 vaccine. The “Routine vaccination” section describes the vaccine recommendations for the general population, while the “Special situations” section describes the vaccine recommendations for persons who are moderately or severely immunocompromised. The number of doses needed and intervals between doses may vary based on a patient’s immunization history, their immunocompromised status, and the vaccine product used.
Haemophilus influenzae type b (Hib) vaccination. Recommendations for Hib vaccination have not changed.
Hepatitis A (HepA) vaccination (4). Minor changes were made to the “Routine vaccination” and “Special situations” sections to improve clarity in the language.
Hepatitis B (HepB) vaccination (5). Additional context was provided in the “Routine vaccination” section to describe the risk-based recommendation for persons 60 years of age and older. The text now reads, “Age 60 years or older without known risk factors for hepatitis B virus infection may receive a HepB vaccine series. Age 60 years or older with known risk factors for hepatitis B virus infection should receive a HepB vaccine series. Any adult age 60 years of age or older who requests HepB vaccination should receive a HepB vaccine series.” A note was added at the end of the “Routine vaccination” section describing the shared clinical decision-making recommendation for persons 60 years of age and older with diabetes.
Human papillomavirus (HPV) vaccination (6). Guidance on interrupted schedules was removed because that information is presented on the cover page. To add clarity, the words “of any valency” were added to the bullet “No additional dose recommended when any HPV vaccine series of any valency has been completed using the recommended dosing intervals.” Lastly, a resource was added to assist health care providers with shared clinical decision-making recommendations for HPV vaccination.
Influenza vaccination (7). For the 2023–2024 influenza season, routine annual influenza vaccination continues to be recommended for all persons aged 6 months and older who do not have contraindications.
The composition of 2023–2024 U.S. influenza vaccines includes an update to the influenza A(H1N1)pdm09 component. All seasonal influenza vaccines available for the 2023–2024 season are quadrivalent. The egg-based vaccines will contain hemagglutinin (HA) derived from an influenza A/Victoria/4897/2022 (H1N1)pdm09–like virus, an influenza A/Darwin/9/2021 (H3N2)–like virus, an influenza B/Austria/1359417/2021 (Victoria lineage)–like virus, and an influenza B/Phuket/3073/2013 (Yamagata lineage)–like virus. The cell culture–based and recombinant vaccines will contain HA derived from an influenza A/Wisconsin/67/2022 (H1N1)pdm09–like virus, an influenza A/Darwin/6/2021 (H3N2)–like virus, an influenza B/Austria/1359417/2021 (Victoria lineage)–like virus, and an influenza B/Phuket/3073/2013 (Yamagata lineage)–like virus.
Bullets referring to having an egg-based allergy were removed from the “Special situations” section, because any influenza vaccine (either egg-based or non–egg-based) indicated for the recipient’s age and health status can be used. A note explaining that any vaccine product appropriate for age and health status can be used for persons with an egg allergy was added at the end of the “Special situations” section.
Measles, mumps, and rubella (MMR) vaccination (8). Minor changes were made to the “Routine vaccination” section to improve clarity in the language.
Meningococcal vaccination (9). Menactra (MenACWY-D) was removed from the Notes section because this product is no longer distributed in the United States. A hyperlink to a resource that describes shared clinical decision making for MenB vaccination is provided. Finally, information on the use of the new pentavalent meningococcal vaccine (MenACWY-TT/MenB-FHbp, Penbraya) was provided at the end of the Meningococcal Notes section.
Mpox vaccination (10). Mpox is a new addition to the Notes section of the adult immunization schedule. Risk factors that warrant routine Jynneos (Bavarian Nordic) vaccination are listed. Bullets about the use of Jynneos among health care providers and in pregnant persons are provided at the end of the Mpox Notes section.
Pneumococcal vaccination (11). Minor edits were made throughout the “Routine vaccination” and “Special situations” sections to provide clarity on the guidance and minimum intervals between doses of pneumococcal vaccines.
Polio vaccination (12). The “Routine vaccination” section was revised and now states that adults who are known or suspected to be unvaccinated or incompletely vaccinated should complete the 3-dose inactivated poliovirus vaccine (IPV) primary series. Additionally, a statement was added stating that most adults born and raised in the United States can assume that they were vaccinated against polio as children. The “Special situations” section describes that adults who are at increased risk for exposure to poliovirus and who have completed the primary series may receive a one-time, lifetime IPV booster dose.
Respiratory syncytial virus (RSV) vaccination (13, 14). RSV is a new addition to the Notes section of the adult immunization schedule. The “Routine vaccination” section describes the use of Abrysvo (Pfizer) during 32 to 36 weeks’ gestation. A sub-bullet was added stating that either maternal RSV vaccination or infant immunization with nirsevimab (RSV monoclonal antibody) is recommended to prevent RSV lower respiratory tract infection in infants. A note was added stating that certain jurisdictions may have RSV seasonality that differs from most of the continental United States and that health care providers should follow guidance from public health authorities on timing of administration based on local RSV seasonality. The “Special situations” section describes the shared clinical decision-making recommendation for vaccination among persons 60 years of age and older; either Abrysvo (Pfizer) or Arexvy (GSK) may be used. A hyperlink to a resource that describes shared clinical decision-making recommendations for RSV vaccination is provided. Finally, a note was added describing the risk factors and medical conditions that health care providers should consider when thinking about a patient’s risk for severe RSV disease and if such patients would benefit from vaccination.
Tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccination (15). A note was added at the end of the Tdap section to clarify that a dose of Tdap received at 10 years of age may be counted as the adolescent dose routinely recommended at age 11 to 12 years.
Varicella vaccination. Routine recommendations for varicella vaccination have not changed.
Zoster vaccination. Routine recommendations for zoster vaccination have not changed.